Menken Monitor centralizes CRA activity, documentation status, visit outcomes, and overdue items into a single source of truth. Line managers and study leaders have a clearer visibility into site performance, reporting timelines, and compliance risks, reducing findings and improving inspection readiness across studies.
Automations reduce repetitive admin tasks like calculating monitoring visit windows, tracking deadlines, chasing reports, and managing follow-ups. CRAs spend less time navigating trackers and emails, and more time focusing on quality monitoring, patient safety, and timely issue resolution.
Built-in checklists, visit guides, templates, and structured workflows help new CRAs get up to speed faster and with fewer mistakes. Menken Monitor standardizes expectations and provides step-by-step support, enabling teams to scale efficiently while maintaining consistent monitoring quality.
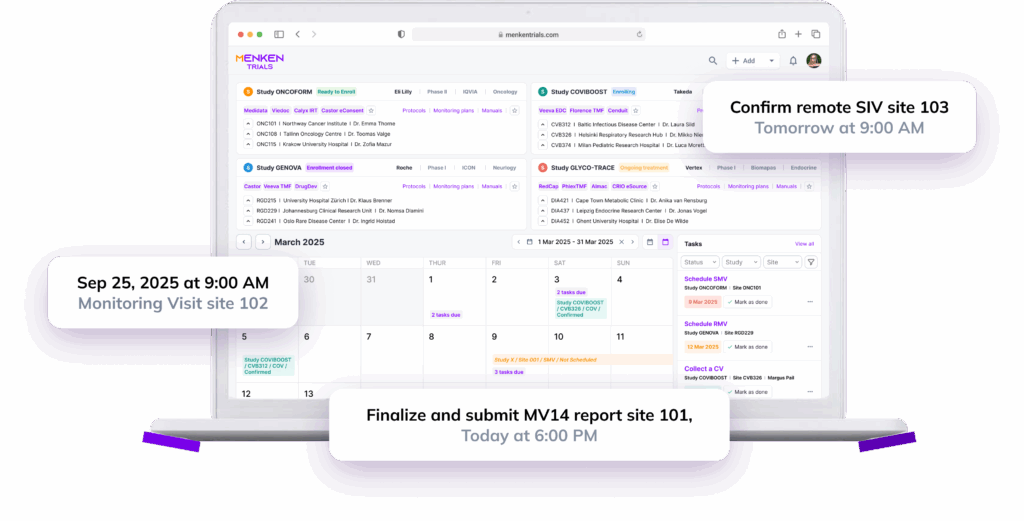

Companies currently wait weeks for monitoring reports and rely on handwritten notes or inconsistent visit summaries. MenkenMonitor gives you live dashboards:
You see problems the moment they appear — not four visits later.

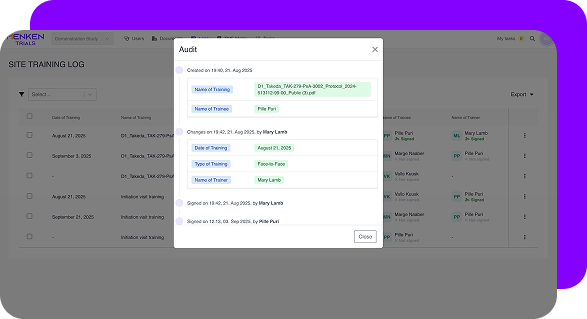
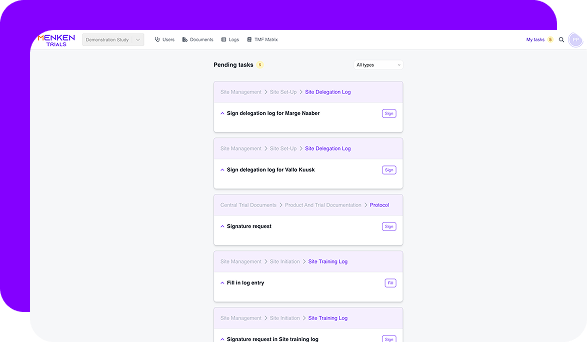
You cannot rely on every CRA having the same experience, discipline, or attention to detail. MenkenMonitor enforces compliance:
This removes variability and protects the company from audit findings caused by human inconsistency.




