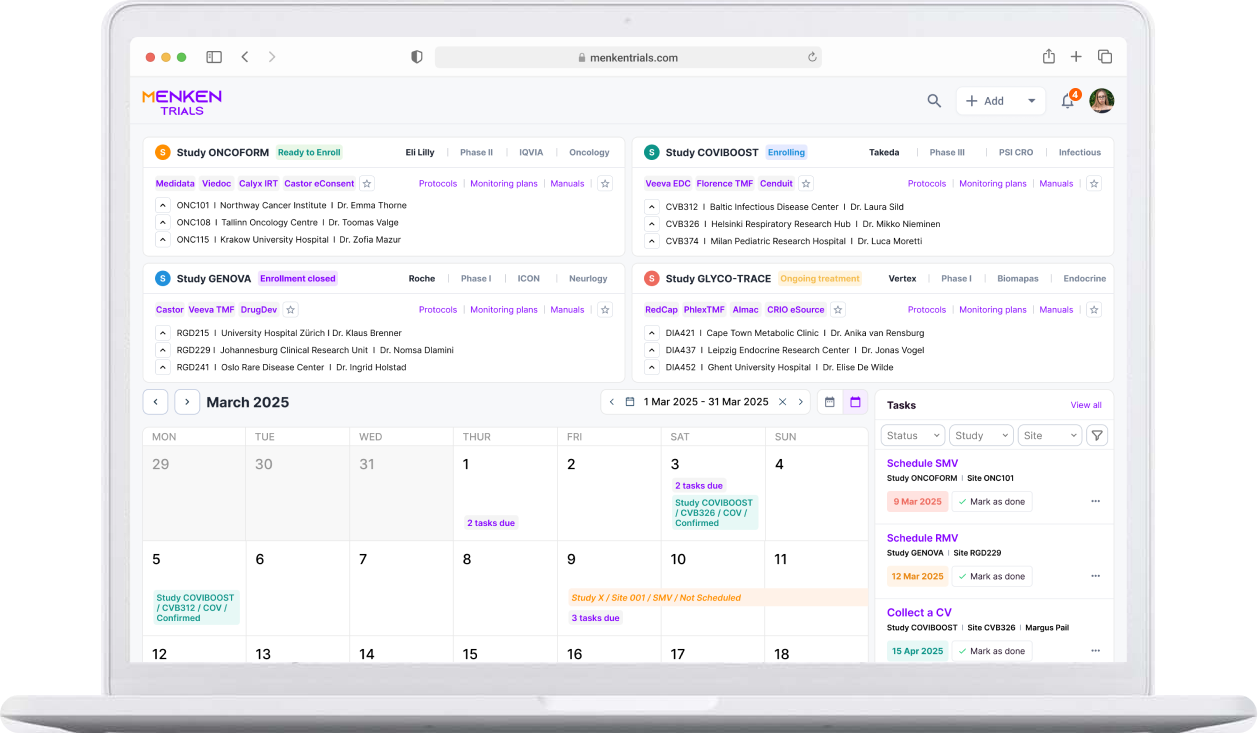
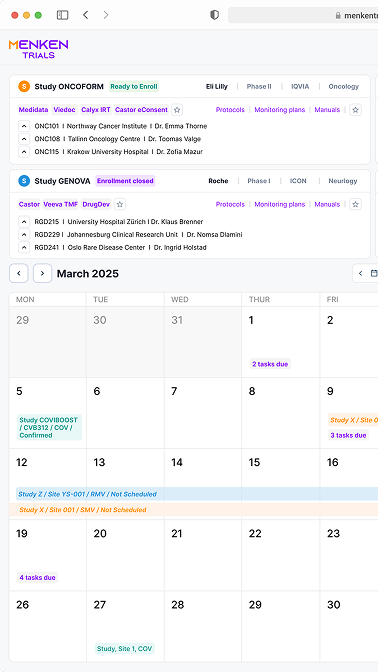


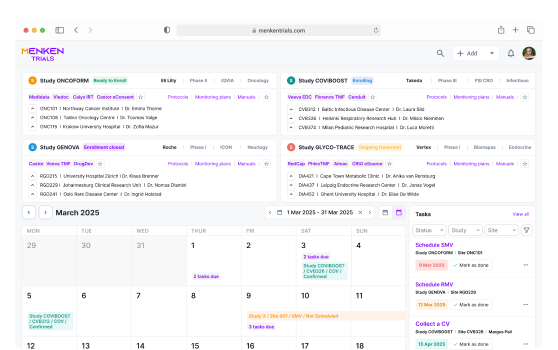
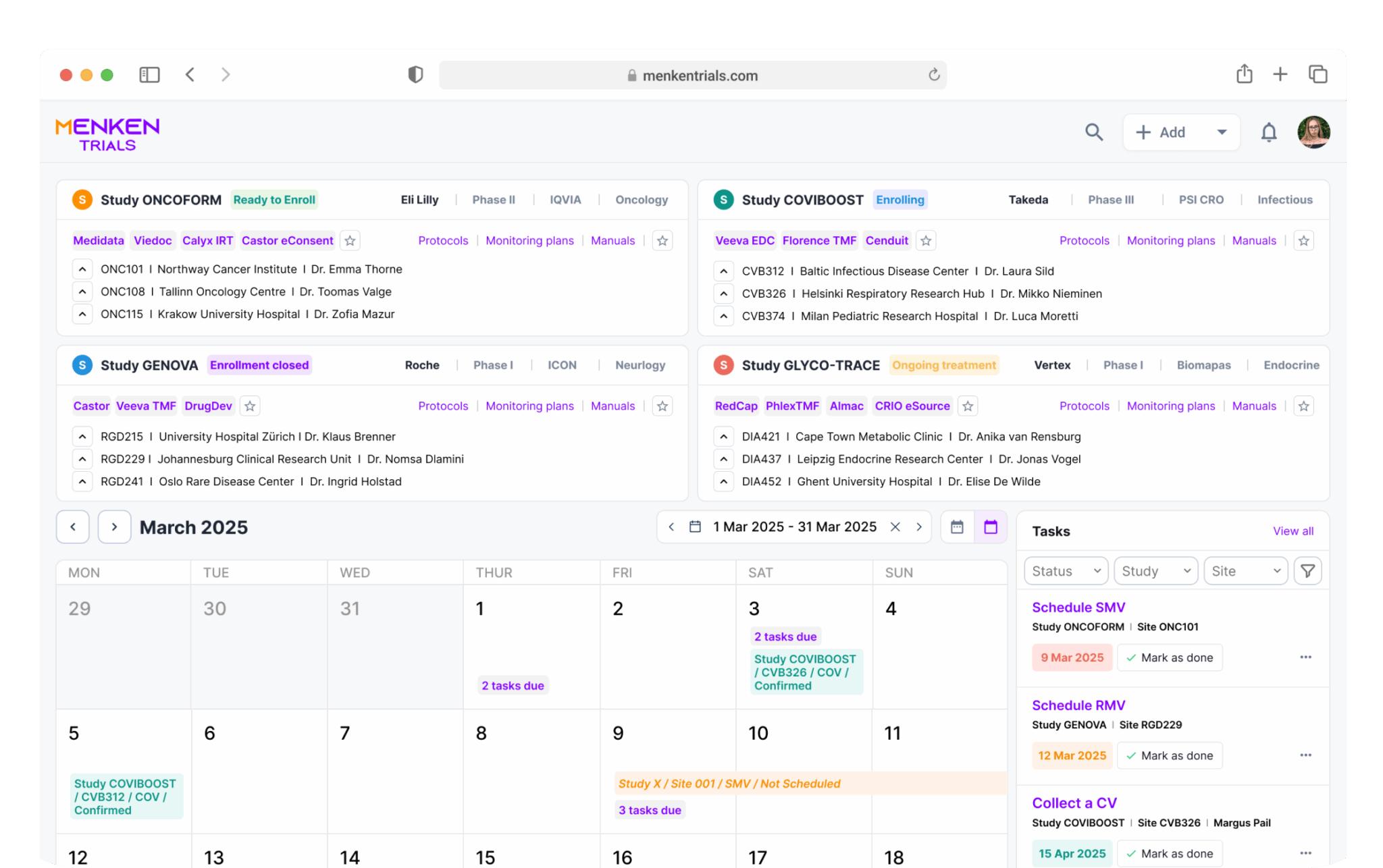
Built-in dashboards show the full status of your clinical trials: from study overview to site status, missing documentation, upcoming monitoring visits and open action items. Easily check what’s overdue, and what needs your attention — all in one place.

Smart checklists, visit templates, and trackers help you plan, execute, and document every visit confidently. Whether you’re preparing for a Routine Monitoring Visit or following up after a Close-Out, Menken Monitor guides you throughout the process.

Menken Monitor flags overdue reports, visit deadlines, missing signatures, and unresolved follow-ups — before they slip through the cracks. Alerts help you stay compliant, avoid surprises, and stay on top of what matters most.







Most Popular
Starter
For getting started
Free
/ per month
Most Popular
Pro
Best for most users
€15.99
/ per month
Most Popular
Elite
For maximum experience
€29.99
/ per month
We’re not just building software — we’re giving CRAs and clinical teams the support we always wished we had. Our goal is simple: to eliminate unnecessary admin, so clinical teams can focus on what truly matters: patient safety, data quality, and trial outcomes.
— Anna-Liisa Parts, Co-Founder & Former CRA