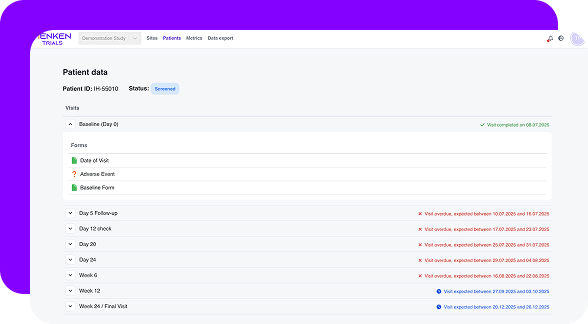




Most inspection findings don’t come from missing documents.
They come from documents that are: outdated, incomplete, approved too late, misaligned with study timelines.
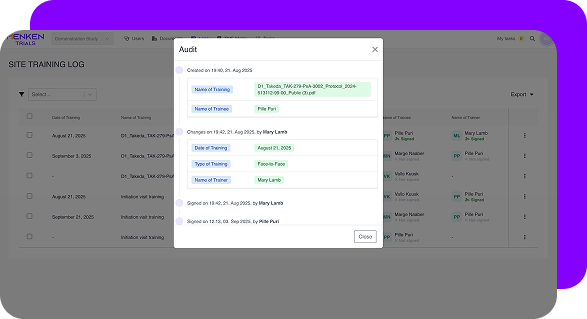
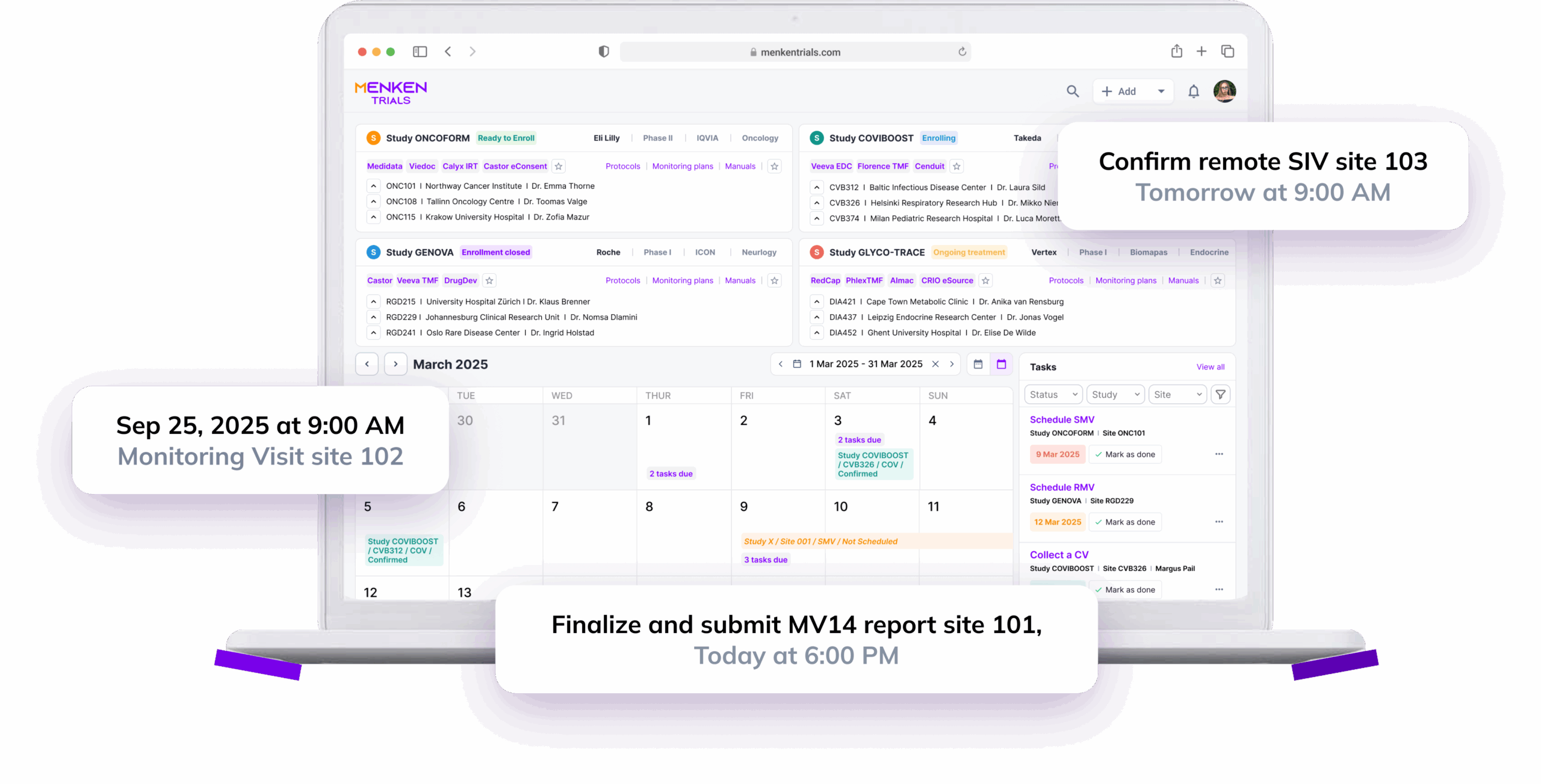
Menken continuously checks essential documents for completeness, versioning, approvals, and timing — so readiness doesn’t drift quietly while studies move forward.
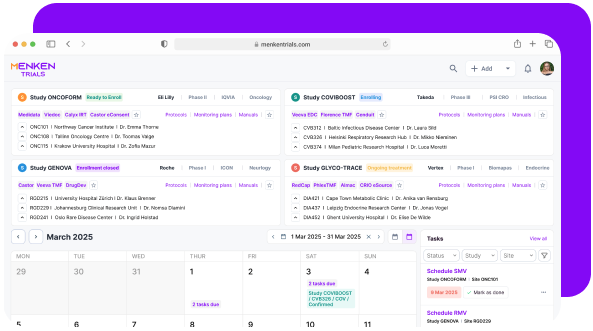
In many studies, risks accumulate silently because no one sees the full picture.
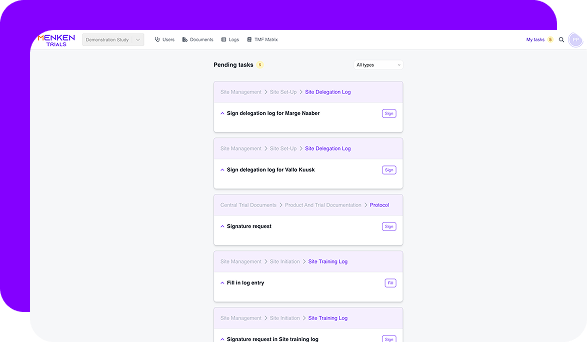
Menken monitors training, essential documents, protocol versions, and commitments together, surfacing gaps early, before they turn into findings, delays, or remediation work.
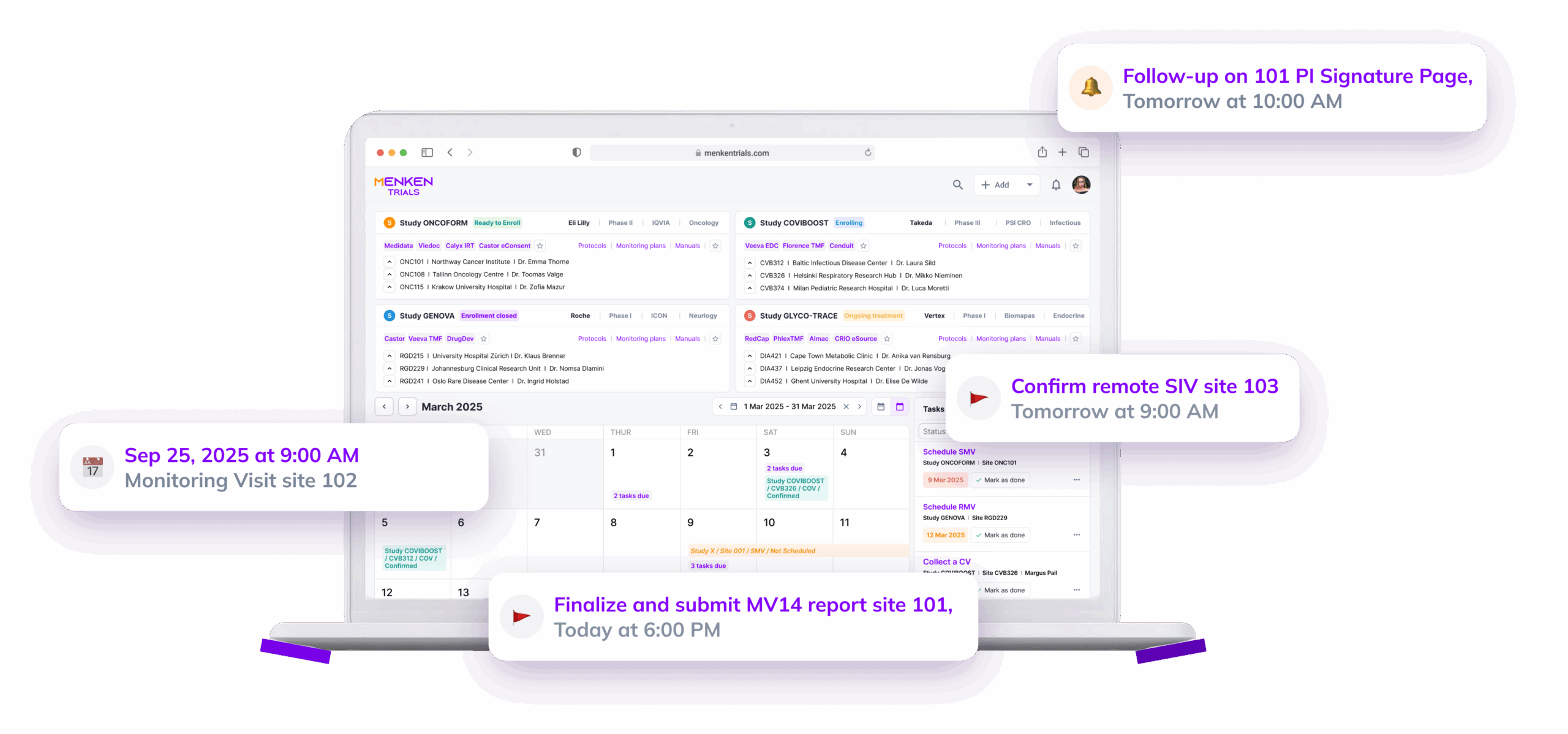
CROs, CRAs, and sites all execute their roles.
But during inspections, accountability sits with the sponsor.
Menken gives sponsors continuous, role-aware visibility across all contributors — so oversight stays central, even when execution is distributed.